MSE 07 Superhydrophobic Coatings Experiment
- Jinwook Chang
- Jan 6, 2024
- 8 min read
Updated: Oct 3, 2025

This experiment creates superhydrophobic surfaces using wax, paraffin, and powders to mimic the lotus effect, demonstrating how surface chemistry and microstructure combine to repel water through bead formation, high contact angles, and self-cleaning behavior.
Introduction
Superhydrophobic surfaces, which are defined by their exceptional ability to repel water, represent an example of how material structure and chemistry can come together to influence material characteristics. Superhydrophobic surfaces are found abundantly in nature, with the most notable instance of this being present in the leaves of the lotus plant. These surfaces exhibit water contact angles exceeding 150°, causing droplets to bead and roll off with minimal resistance. This unique behavior is the result of the surface's microscopic and nanoscopic architecture combined with low surface resistance. In industrial and research contexts, superhydrophobic coatings have become a subject of intense interest for applications ranging from self-cleaning building materials to anti-icing surfaces in aviation and marine applications. Achieving this effect typically requires precise fabrication and formulation of both micro and nanostructures, and the application of low-resistance surface treatments. While these processes can involve advanced equipment such as plasma etchers, chemical vapor deposition systems, or nanolithography, it is also possible to replicate the result using simpler techniques, such as the one that will be used for this experiment.

This experiment highlights the key principles behind superhydrophobicity using accessible materials such as candle wax, paraffin, and commercially available hydrophobic sprays. By modifying the surfaces of various substrates such as glass, metal, or plastic, we can simulate the lotus effect in a form that is still visibly stunning. Through preparation, coating, and droplet testing, we can modify the surface of any material to become superhydrophobic.
Aim
This experiment aims to create and evaluate surfaces with superhydrophobic properties by applying hydrophobic coatings combined with fine powders to simulate micro-structural roughness. The objectives of this experiment are:
To demonstrate the influence of surface chemistry and structure on water-repelling behavior.
To compare the performance of wax-based and paraffin treatments on different substrate materials.
To observe the role of added powders in enhancing ultrahydrophobicity through creating microstructures.
By the end of the experiment, we will be able to identify the visual and measurable indicators of ultrahydrophobicity, including bead formation, high contact angles, and rapid droplet roll-off, as well as understand the underlying chemical properties of superhydrophobic coatings.
Context
The lotus leaf effect, first studied in detail in the late 20th century, has become a benchmark in surface engineering. In the natural world, lotus leaves remain clean despite constant exposure to muddy water because their surfaces are covered in microscopic papillae coated with hydrophobic waxes. These features result in a rough outer coating. The micro-scale bumps covered in nanoscale wax crystals serve the purpose of trapping air and reducing the solid-liquid contact area, causing water droplets to sit almost entirely on air pockets, which means they easily roll off the leaf.

This phenomenon inspired the creation of artificial superhydrophobic surfaces. In research laboratories, materials like silica nanoparticles, polymers, and etched metals have been designed to replicate these effects. Commercial applications now include water-resistant clothing, anti-fouling ship hulls, wind turbine blades with ice-shedding coatings, and even certain surgical-grade medical devices that resist bacterial adhesion. The simplified approach in this experiment utilizes these concepts in an accessible and reproducible way. Whilst our methodology will not match the durability, chemical resistance, or nanostructural precision of advanced industrial coatings, it can still demonstrate the core concept, with that being that by altering both the chemistry and the topography of a surface, we can drastically change how it will interact with water.

In addition to the lotus leaf, several other plants and insects display natural ultrahydrophobicity. For example, the wings of cicadas and dragonflies have nano-structured surfaces that not only repel water but can also kill bacteria upon contact. This combination of self-cleaning and antimicrobial properties has inspired the next generation of coating and is making its way into hospitals and showing promising medical applications, where sterility is paramount. Similarly, certain desert beetles use patterned hydrophobic and hydrophilic areas on their backs to collect and channel water from fog, an adaptation that is now being mimicked in water-harvesting technologies in rural mountainous regions where water scarcity is an issue. Super hydrophobicity has a functional use case with survival benefits, making it a particularly valuable creation for bio-inspired engineering.

Theory
Wettability, which is defined as the degree to which a liquid rolls on or adheres to a solid, is measured by the contact angle, which is the angle between the liquid droplet and the solid surface at their contact line. A surface is considered hydrophobic if the contact angle is greater than 90°, and superhydrophobic if it exceeds 150°. In our experimental coatings, wax or paraffin reduces the surface energy, while the applied powders mimic micro-structural features, creating air pockets beneath droplets and enhancing water repellency.
By combining these elements, we can create surfaces that cause droplets to roll off, cleaning the surface in the process – a self-cleaning behavior with wide-ranging use cases.
Materials
Base material (for the surface):
Glass slides, plastic sheets, or metal pieces.
Hydrophobic substance:
Candle wax or paraffin wax.
Alternative: Water-repellent sprays (e.g., silicone spray or NeverWet).
Fine powder:
Talcum powder, Teflon powder, or silica powder (to mimic microstructures).
Heat source:
Hairdryer, heat gun, or lighter.
Tools:
Tweezers or tongs (to handle materials).
Brush or sponge (for applying wax or spray).
Water dropper:
For testing water behavior on the coated surface.
Optional testing tools:
Protractor or smartphone app (to measure the contact angle of water).
Procedure
Step 1: Prepare the Base Surface
Clean the Surface:
Wash the glass, plastic, or metal surface with soap and water to remove dirt or grease.
Dry thoroughly to ensure proper coating adhesion.
Roughen the Surface (Optional):
Use fine sandpaper to create a slightly rough texture, which enhances hydrophobic effects.
Step 2: Apply the Hydrophobic Layer
Method 1: Using Candle Wax
Melt the Wax:
Heat a candle or paraffin wax over a heat source until it starts melting.
Coat the Surface:
Dip the base material into the melted wax or use a brush to evenly apply a thin layer.
Dust with Powder:
While the wax is still warm, sprinkle a fine powder (e.g., talcum or silica powder) onto the surface. This mimics the microstructures found on lotus leaves.
Set the Wax:
Let the wax cool and harden for a few minutes.
Method 2: Using Water-Repellent Spray
Spray the Surface:
Apply an even layer of water-repellent spray to the surface, holding the can 10–15 cm away.
Dust with Powder (Optional):
Add a layer of fine powder to enhance the microstructures.
Dry the Surface:
Allow the spray to dry for 10–15 minutes before testing.
Step 3: Test the Superhydrophobic Coating
Water Droplet Test:
Use a dropper to place a small water droplet on the treated surface.
Observe how the droplet behaves—on a superhydrophobic surface, it should bead up and roll off easily.
Contact Angle Measurement:
Use a protractor or smartphone app to measure the angle between the droplet and the surface. A contact angle greater than 150° indicates superhydrophobicity.
Tilt Test:
Tilt the surface to check how quickly the droplet slides off.
Observations
Water Behavior:
On a successful superhydrophobic surface, water droplets should bead up and slide off with minimal resistance.
Durability:
Test the coating by gently rubbing the surface or exposing it to water multiple times to see if the hydrophobic properties persist.
Analysis
Effect of Powder:
Compare the hydrophobicity of surfaces with and without added powder to see the impact of microstructures.
Different Substrates:
Test the coating on various materials (glass, plastic, metal) to evaluate performance differences.
Optimization
Layer Thickness:
Experiment with thicker or thinner layers of wax or spray to optimize performance.
Applications
Learn the science behind water-repellent coatings used in textiles, electronics, and self-cleaning surfaces.
Explore potential applications in preventing corrosion or making surfaces easier to clean.
Qualities of the Coated Surfaces
The coated surfaces prepared in this experiment exhibit several key qualities characteristic of ultrahydrophobicity:
High contact angle: Beads are tall and nearly spherical.
Low sliding angle: Droplets roll off with a slight inclination of the surface.
Self-cleaning effect: Dust and debris are carried away as droplets roll, leaving a clean track.
Texture influence: Surfaces with well-distributed powder display more consistent superhydrophobic behavior.
These qualities correlate directly with the degree of surface roughness and the hydrophobicity of the coating material used.
Economic and Environmental Factors
From an economic point of view, wax-based coating is inexpensive, requiring only a few materials, with those being: a common candle and household powders, all of which can be obtained fairly cheaply and easily. Environmentally, wax coatings present minimal hazard, being biodegradable and easy to remove. Talcum powder and silica are inert but can pose inhalation hazards if handled improperly. In industry, advanced superhydrophobic coatings are produced using energy-intensive nanofabrication techniques. The simplified experiment, therefore, offers an accessible way to demonstrate the phenomenon without the same energy footprint.
Applications
Educational applications of this experiment are plentiful, including demonstrations of wetting theory and the effects of microstructure on a macroscopic scale. In real-world contexts, superhydrophobic coatings are used in:
Textiles: To produce stain-resistant, waterproof fabrics.
Electronics: To protect circuitry from water ingress.
Architecture: For self-cleaning windows that don’t collect water droplets
Marine engineering: To reduce drag and biofouling on ship hulls.
Aerospace: To prevent ice formation on wings and turbine blades.
Furthermore, in the biomedical field, superhydrophobic surfaces are being researched for their potential to reduce biofilm formation on implants and surgical tools. By preventing the adhesion of water and fluids, these surfaces can reduce the risk of infection. In renewable energy, solar panels with superhydrophobic coatings can maintain high efficiency by preventing dust and dirt accumulation without the need for frequent cleaning, which is particularly beneficial in arid and dusty environments.
Chemical Properties and Structure
The hydrophobic effect in these coatings arises from a combination of low-surface-energy chemistry and engineered surface structure. Wax consists of long-chain hydrocarbons, which are non-polar and therefore repel polar water molecules. Commercial sprays often deposit thin films of silicone, which have extremely low surface energies.
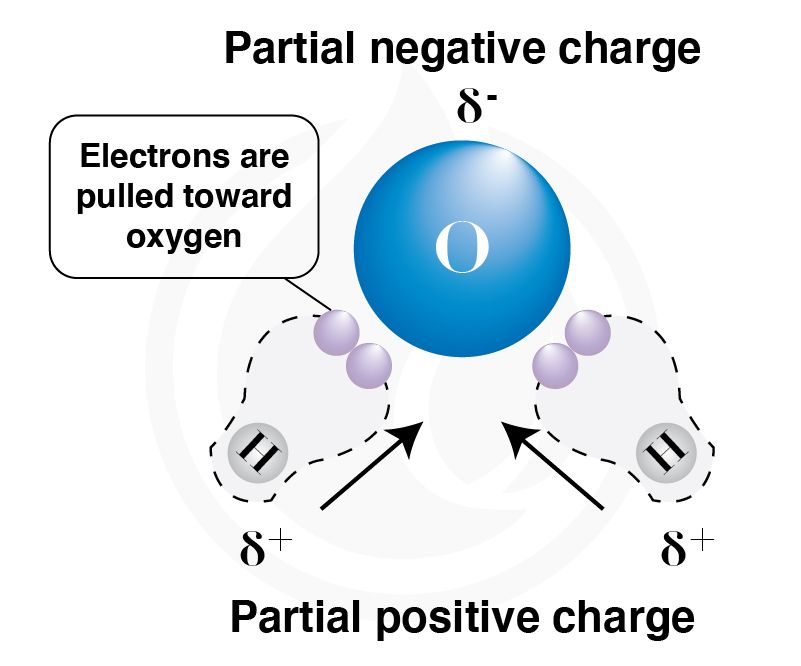
Hydrophobicity and hydrophilicity arise from how molecules interact with water’s polarity. A water molecule is polar because oxygen is more electronegative than hydrogen, creating a partial negative charge (δ–) on oxygen and partial positive charges (δ+) on the hydrogens. Hydrophilic substances have polar groups (like –OH, –NH₂, –COOH) that can form hydrogen bonds with water, making them dissolve or mix easily. Hydrophobic substances, like hydrocarbons, lack polarity and cannot form favorable interactions with water, so they cluster together to minimize contact, leading to effects like droplet beading or phase separation.
The powders add a physical component, as microscopic particles increase roughness and create air pockets beneath the water droplets. In natural lotus leaves, this combination of hydrophobic chemistry and microstructure leads to ultrahydrophobicity. In our artificially made surfaces, although the scale of roughness is much larger and less uniform than in nature, the underlying principle remains the same.
Conclusion
This experiment demonstrates the creation of superhydrophobic surfaces using readily available materials. Both wax and paraffin application methods produce significant increases in contact angle compared to untreated substrates, with the addition of fine powders having the ability to enhance performance by introducing micro-structural roughness. Through visual and quantitative testing, we can observe how chemistry and topography combine to influence wetting behavior. This experiment reinforces theoretical concepts such as contact angle measurement and the relationship between surface structure and the ability to repel water. By understanding the factors that control wettability, we can appreciate how small-scale structures influence large-scale behaviors and how these principles can be applied to real-life challenges such as infrastructure maintenance











Mình có lần lướt đọc mấy trao đổi trên mạng thì thấy nhắc tới bongdaso.org trong lúc mọi người đang bàn luận về kết quả các trận đấu, nên cũng mở ra xem thử cho biết. Mình không tìm hiểu sâu, chỉ xem qua trong thời gian ngắn để nhìn cách bố trí thông tin, tỷ số và các mục liên quan. Cảm giác là trình bày khá gọn, các phần rõ ràng nên đọc lướt cũng không bị rối, với mình như vậy là đủ để nắm thông tin cơ bản rồi trên bongdaso.org.