FSE 03 Exploring the Influence of pH on Yeast Bead Movement in Hydrogen Peroxide
- Jinwook Chang
- Nov 3, 2023
- 7 min read

This experiment investigates how different pH levels affect the activity of catalase in yeast by observing the movement of yeast beads in hydrogen peroxide. By tracking bead motion as oxygen is released, students can visualize enzyme activity and better understand how pH influences biochemical reactions.
Abstract
Enzymes are specific examples of proteins and are therefore made up of polypeptide chains (chains of amino acids). As biological catalysts, enzymes facilitate the acceleration of chemical reactions.

In this laboratory experiment, I will explore the functionality of a particular enzyme known as catalase, which is present in yeast. Catalase is responsible for decomposing Hydrogen peroxide (H2O2)—a waste product of metabolism in cells—into water and oxygen, as indicated by the following reaction:

As proteins, enzymes function is determined by their structure. Changing the conditions under which the enzyme works can influence its structure and therefore its function. Such condition includes temperature and pH.
In this laboratory investigation, I will assess the catalase activity at various pH levels, examining the influence of the addition or omission of the hydrochloric acid.
Introduction
Research Question
How does changing the pH of the Yeast bead environment affect the length of time for the Yeast bead to rise to the top of the hydrogen peroxide solution?
Hypothesis
If the pH of the Yeast beads environment changes from the optimal pH, which is predicted to be around 7, then the time(s) for Catalase to break down the hydrogen peroxide will increase since the enzyme will denature, changing the shape of the active site, which no longer will fit the substrate hydrogen peroxide
Independent, Dependent, Controlled Variables
Independent Variable
Changing the pH of the Yeast beads environment (pH-2 HCl, pH-6.5 H2O, pH-13 NaOH)
Dependent Variable
The time it takes for a Yeast bead to rise to the top of the hydrogen peroxide (s)
Controlled Variable
Controlled Variable/Constant | Why must the variable be controlled | How will the variable be controlled |
1) Height from which the bead is dropped | If the height is increased, then the reaction time increases. | The Yeast bead will be released using forceps even with the lip of the measuring cylinder. |
2) Length of time the Yeast bead is soaking in the different pH environment | If the soaking time is increased, then the reaction time will increase because the active site has denatured. | East Yeast bead will sit in the different pH solutions for 1 minute. |
3) Volume and concentration of hydrogen peroxide in the measuring cylinder | If the volume is increased, the reaction time will increase. If the concentration is increased, then the reaction time will decrease. | The measuring cylinder with hydrogen peroxide will be kept at volume of 50mL and at the same concentration. |
4) Maintaining a constant temperature for all treatments and trials | If temperature decreases, the reaction rate could decrease as the molecules would not collide. | All materials and solutions will be kept at room temperature before and during the experiment to ensure room temperature is constant. |
Other Relevant Controlled Variables:
1) Moment at which the stopwatch is started and when it is stopped
2) Yeast beads will not be reused
3) Shape and size of the measuring cylinder
Equipment
Sodium alginate and yeast beads
1% Hydrogen peroxide (H2O2)
Hydrochloric Acid (HCl) 1%
Sodium Hydroxide (NaOH) 0.5%
Measuring cylinder (50 mL)
Beakers
pH strips
Stopwatch
Forceps
Methodology
Obtain three beakers
Place 5 sodium alginate and yeast beads into each beaker
Submerge the beads in one beaker with water (pH around 7)
Submerge the beads in the other beaker with hydrochloric acid (HCl) (pH around 2)
Submerge the beads in the other beaker with sodium hydroxide (NaOH) (pH around 13)
Measure and record the pH of each beaker
Place 50mL of H2O2 into the measuring cylinder.
Gently using forceps, drop one Yeast bead into the hydrogen peroxide and start the timer.
Time how long it takes for the bead to rise back to the top of the measuring cylinder. The catalase will be reacting with the hydrogen peroxide, and the oxygen produced will cause the bead to rise.
Remove the used bead and discard it in the trash.
Using a new bead each time, repeat steps 3-6 a minimum of 5 times (maximum 10). The hydrogen peroxide can be reused. If necessary, top up the hydrogen peroxide to 50mL.
Repeat steps 3-7 using the beads that were treated with hydrochloric acid.
Repeat steps 3-7 using the beads that were treated with sodium hydroxide.
Data Analysis
Table 01: Time Taken for Yeast Balls to Rise in Treatments using Different Substances with Different pH

Table 02: Average time taken for yeast balls to rise in treatments using different substances with different pH levels

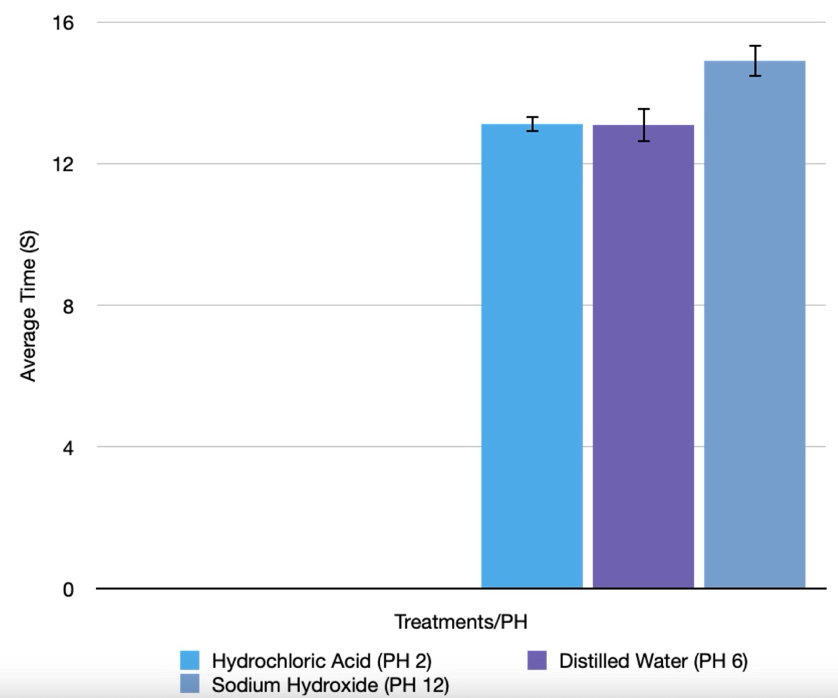
Graph 01: Time Taken for Yeast Balls to Rise in Treatments using Different Substances with Different pH levels
Qualitative Observations
HCI: The yeast beads stayed under the H202 surface for a considerable duration. At times, they adhered to the cylinder walls.
H2O: The yeast beads stayed under for a shorter span and moved up quicker upon resurfacing. The H2O emitted an odd odor, suggesting possible contamination from insufficiently cleaned beakers or residual chemicals. Additionally, there might have been minor errors in timing the yeast beads' resurfacing, potentially starting the stopwatch a bit too early or late.
NaOH: The beads remained submerged for extended periods but resurfaced with greater speed. Like with HCI, the beads sometimes stuck to the cylinder walls.
Conclusion
Claim
Through experiments assessing the yeast beads’ capacity to ascend in the solution under three distinct treatments—Hydrochloric Acid, Distilled Water, and Sodium Hydroxide—each characterized by its specific pH level, we determined that the pH level having the most significant impact on accelerating the bead’s accent in the shortest duration was pH 6.5, corresponding to Distilled Water, strongly supporting my estimation in the hypothesis.
Evidence
For the initial treatment (Hydrochloric Acid) with a pH level 2, the yeast bead took an average of 13.122 seconds to ascend to the top of the solution. In contrast, the second treatment (Distilled Water), featuring the optimal pH level of approximately 7 (6.5), showed a faster average ascent time of only 13.086 seconds for the yeast bead. Additionally, the third treatment (Sodium Hydroxide) with a pH level of 12 exhibited a longer average ascent time compared to water, clocking in at 14.898 seconds for the yeast beads’ ascent.
Moreover, the error bars of the average Catalase reaction time do not overlap in Sodium Hydroxide and Distilled Water. The error bars also do not overlap in Hydrochloric Acid and Sodium Hydroxide. However, the error bars of the average Catalase reaction do overlap in Hydrochloric Acid and Distilled Water.
Explanation
The collected data reveals a clear pattern indicating that the farther the pH level deviates from the optimal value of 7, the longer it takes for the yeast bead to ascend. This phenomenon is rooted in biological principles, as enzymes, functioning as catalysts in chemical reactions, operate optimally within specific conditions, including pH levels. When these factors are not at their optimal levels, enzymes may undergo denaturation, causing a significant change in the active site’s shape and rendering it incompatible with the substrate H2O2. Indeed, the data supports this concept, showing that at the pH level of 6.5, the more or less optimal condition, the yeast bead exhibited the fastest average rising time of 13.086 seconds. Conversely, at pH levels 2 and 12 in Hydrochloric Acid and Sodium Hydroxide, Catalase reaction times was slower, leading to the slower rising time of the yeast beads. Hence, this highlights the enzyme’s sensitivity to the environment’s pH level, with deviations leading to signs of denaturation, as observed in the test of Sodium Hydroxide.
Moreover, the error bars of the average Catalase reaction time do not overlap in Sodium Hydroxide and Distilled Water. This implies that there’s a statistically significant difference between the data points they represent, reinforcing the claim of the optimum pH level of the Catalase reaction being closer to a neutral pH level (6.5 in this experiment). However, the error bars of the average Catalase reaction do overlap in Hydrochloric Acid and Distilled Water. Though when the error bars represent standard deviation, which measures the spread of the data around the mean, overlapping error bars are less informative about statistical significance. Overlap in this case is more about the variability of the data and does not necessarily imply that the means are not significantly different.
(There probably was a mistake measuring the times for trial 1 and trial 2 for the experiments regarding Hydrochloric Acid, as they were our first two experiments when we were not too familiar with what we were doing.)
Evaluation
Limitations
Reaction Time Variability: (This affected the Hydrochloric Acid experiment for trials 1 and 2)
The initiation of the timer when dropping the yeast beads into the test tubes was subject to natural variations in human reaction time. This inherent variability introduces an element of unfairness to the experiment as the timer could be triggered slightly earlier or later.
Variation in Yeast Beads:
In this experiment, 15 different yeast beads were utilized where each yeast bead somehow possessed a unique characteristic such as weight, size, and shape, making the experiment slightly challenging to replicate precisely.
Temperature Variation:
The experiment was significantly influenced by the room temperature, a crucial factor that was not consistently monitored. The temperature might have fluctuated during the experiment, potentially rendering the test unfair.
Improvements
To address the initial issue, a potential solution could involve implementing a machine or artificial intelligence programmed to activate a timer immediately upon detecting any contact between the yeast bead and the water. It's worth noting that current technology may not fully support this approach at the moment.
To overcome the second constraint, it is conceivable to meticulously measure each yeast bead, considering factors such as size, weight, and dimensions. This meticulous approach ensures a thorough verification of the uniformity and equality of the yeast beads.
To address the last limitation, we could experiment in a controlled environment where the temperature remains constant throughout. This could be executed in a storage room or classroom devoid of external temperature-altering factors such as windows, air conditioning, or heating. Observing the impact of a stable temperature on the experiment would help mitigate this weakness.




Comments